Proton donor in chemistry crossword clue – Embarking on the topic of proton donors in chemistry, this comprehensive guide delves into the fundamental concepts, reactions, and applications of proton donors, equipping readers with a thorough understanding of this critical aspect of chemistry.
Proton donors play a pivotal role in numerous chemical processes, including acid-base reactions, proton transfer reactions, and acid-base equilibria. Understanding their behavior and properties is essential for solving crossword clues related to proton donors and gaining a deeper appreciation for their significance in chemistry.
Proton Donors in Chemistry: Proton Donor In Chemistry Crossword Clue

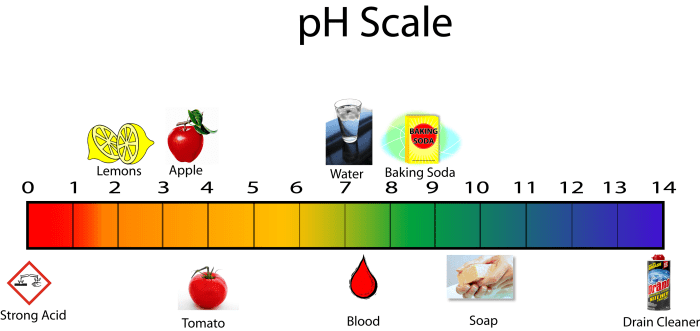
In chemistry, a proton donor is a substance that can donate a proton (H+ ion) to another substance. Proton donors are typically acids, which are substances that can release H+ ions in water. The ability of a proton donor to donate protons is determined by its acidity, which is measured by its pH.
The lower the pH of a solution, the more acidic it is and the more protons it can donate.
Acids
Acids are substances that can donate protons. The strength of an acid is determined by its ability to donate protons. Strong acids, such as hydrochloric acid (HCl) and sulfuric acid (H2SO4), can donate protons easily. Weak acids, such as acetic acid (CH3COOH) and carbonic acid (H2CO3), can donate protons less easily.
Proton Transfer Reactions
Proton transfer reactions are chemical reactions in which a proton is transferred from one substance to another. Proton transfer reactions are typically acid-base reactions, in which an acid donates a proton to a base. The strength of a proton donor is determined by its ability to donate protons.
Strong proton donors can donate protons easily, while weak proton donors can donate protons less easily.
Acid-Base Equilibria, Proton donor in chemistry crossword clue
Acid-base equilibria are chemical equilibria in which an acid and a base react to form a conjugate acid-base pair. The equilibrium constant for an acid-base reaction is determined by the strength of the acid and the base. Strong acids and bases have large equilibrium constants, while weak acids and bases have small equilibrium constants.
pH and Proton Concentration
The pH of a solution is a measure of its acidity. The pH of a solution is determined by the concentration of protons in the solution. The lower the pH of a solution, the higher the concentration of protons in the solution.
Proton donors can increase the concentration of protons in a solution, thereby decreasing the pH of the solution.
FAQ Insights
What is the role of proton donors in acids?
Proton donors are substances that can release protons (H+ ions) into a solution, giving acids their characteristic properties.
What is a proton transfer reaction?
A proton transfer reaction is a chemical reaction in which a proton is transferred from one molecule or ion to another.
How does proton donor strength affect the pH of a solution?
Stronger proton donors release protons more readily, leading to a lower pH (more acidic) solution.