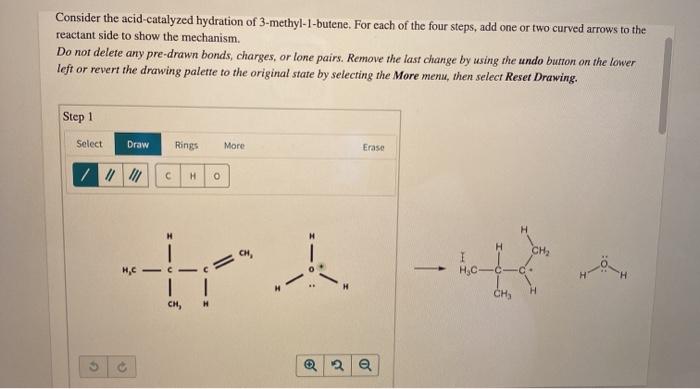
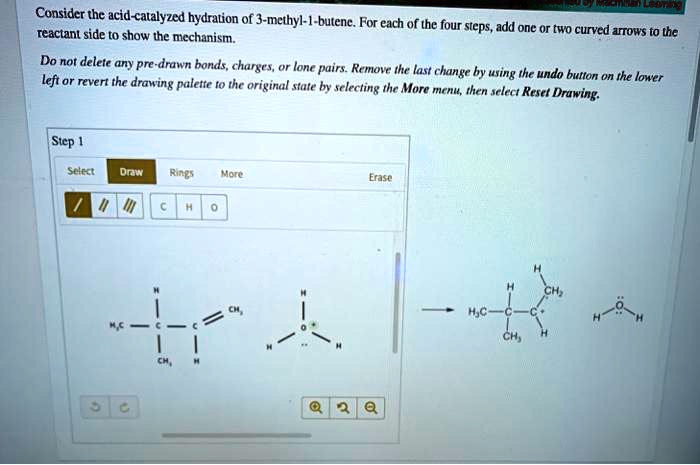
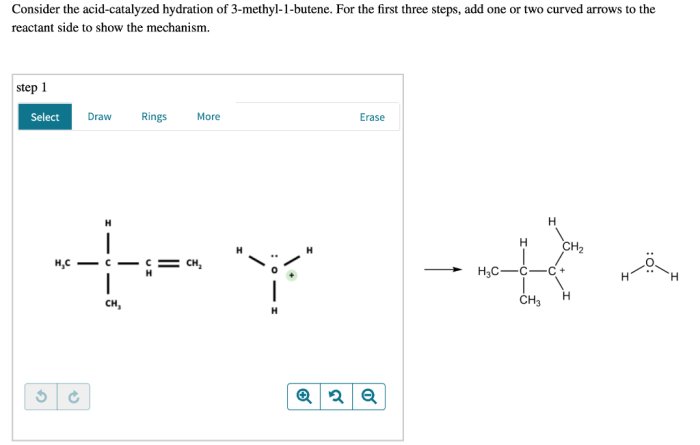
As we delve into the captivating realm of organic chemistry, consider the acid-catalyzed hydration of 3-methyl-1-butene, a reaction that unveils a symphony of intricate mechanisms and profound applications. This comprehensive analysis embarks on a journey to unravel the complexities of this reaction, shedding light on its regioselectivity, stereochemistry, and practical significance.
The acid-catalyzed hydration of 3-methyl-1-butene, a fundamental reaction in organic chemistry, provides a rich tapestry of insights into the behavior of organic compounds. By examining the detailed chemical equation and step-by-step mechanism, we uncover the intricacies of electrophile-nucleophile interactions and the role of the acid catalyst.
Acid-Catalyzed Hydration of 3-Methyl-1-butene
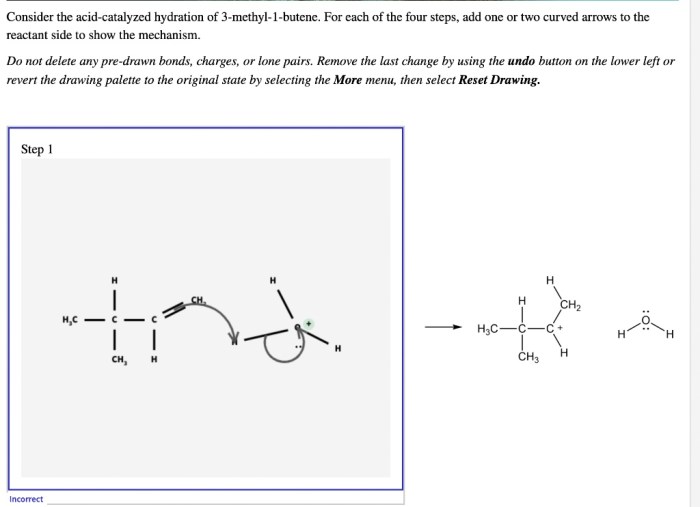
The acid-catalyzed hydration of 3-methyl-1-butene is a chemical reaction that adds water to the double bond of the alkene, forming an alcohol. The reaction is typically carried out in the presence of a strong acid, such as sulfuric acid or hydrochloric acid.
The chemical equation for the reaction is as follows:
-methyl-1-butene + H2O → 2-methyl-2-butanol
Reaction Mechanism
The reaction mechanism for the acid-catalyzed hydration of 3-methyl-1-butene is a two-step process. In the first step, the acid protonates the double bond of the alkene, forming a carbocation. In the second step, the water molecule attacks the carbocation, forming an alcohol.
The electrophile in the reaction is the carbocation, and the nucleophile is the water molecule. The acid catalyst plays a role in the reaction by protonating the double bond and making it more reactive.
Regioselectivity
The reaction is regioselective, meaning that it favors the formation of the more substituted carbocation. This is because the more substituted carbocation is more stable than the less substituted carbocation.
Markovnikov’s rule states that in the addition of a protic acid to an unsymmetrical alkene, the hydrogen atom of the acid adds to the carbon atom of the double bond that has the most hydrogen atoms.
Stereochemistry
The reaction is stereospecific, meaning that the stereochemistry of the starting material determines the stereochemistry of the product.
If the starting material is a cis-alkene, the product will be a threo-diol. If the starting material is a trans-alkene, the product will be an erythro-diol.
Applications, Consider the acid-catalyzed hydration of 3-methyl-1-butene
The acid-catalyzed hydration of 3-methyl-1-butene is a useful reaction in organic synthesis. It can be used to prepare a variety of alcohols, including 2-methyl-2-butanol, which is used as a solvent and a fuel additive.
The reaction is also used in the industrial production of 2-methyl-2-butanol. The reaction is carried out in a continuous reactor, and the product is separated by distillation.
User Queries: Consider The Acid-catalyzed Hydration Of 3-methyl-1-butene
What is the significance of regioselectivity in the acid-catalyzed hydration of 3-methyl-1-butene?
Regioselectivity plays a crucial role in determining the orientation of the water molecule’s addition to the double bond. Markovnikov’s rule governs this regioselectivity, favoring the formation of the more substituted carbocation, leading to the predominant formation of 2-methyl-2-butanol.
How does the stereochemistry of the starting material influence the product in the acid-catalyzed hydration of 3-methyl-1-butene?
The stereochemistry of the starting material dictates the stereochemistry of the product. If the starting material is a cis-alkene, the product will be a mixture of diastereomers. Conversely, if the starting material is a trans-alkene, the product will be a single enantiomer.