The answer key types of chemical bonds worksheet is an invaluable resource for students seeking to master the fundamentals of chemical bonding. This worksheet provides a comprehensive overview of the various types of chemical bonds, their properties, and their applications.
Chemical bonds are the forces that hold atoms together to form molecules and compounds. Understanding the different types of chemical bonds is crucial for comprehending the structure and behavior of matter.
Types of Chemical Bonds
Chemical bonds are forces that hold atoms together to form molecules and compounds. There are several types of chemical bonds, each with its own unique characteristics.
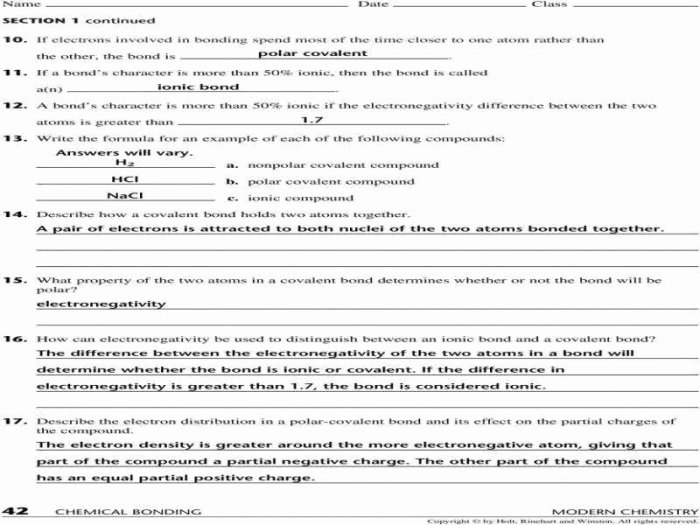
Covalent Bonds, Answer key types of chemical bonds worksheet
- Formed when atoms share electrons
- Strong and nonpolar
- Example: HCl
Ionic Bonds
- Formed when atoms transfer electrons
- Strong and polar
- Example: NaCl
Hydrogen Bonds
- Formed between a hydrogen atom and a highly electronegative atom (such as oxygen, nitrogen, or fluorine)
- Weak and polar
- Example: H 2O
Metallic Bonds
- Formed between metal atoms
- Strong and nonpolar
- Example: Copper
Properties of Chemical Bonds: Answer Key Types Of Chemical Bonds Worksheet

The properties of a chemical bond determine the properties of the molecule or compound it forms.
Strength
The strength of a bond is measured by its bond energy, which is the amount of energy required to break the bond.
Polarity
The polarity of a bond is determined by the difference in electronegativity between the atoms involved. A polar bond has a partial positive charge on one atom and a partial negative charge on the other atom.
Length
The length of a bond is the distance between the nuclei of the atoms involved.
Bond Formation

Bond formation occurs when atoms or molecules come close enough to each other to share or transfer electrons.
Electrons are negatively charged particles that are found in the outer shells of atoms. When atoms share electrons, they form a covalent bond. When atoms transfer electrons, they form an ionic bond.
Bond Breaking
Bond breaking occurs when the atoms or molecules involved in the bond are pulled apart.
There are several ways to break a bond, including:
- Heating
- Adding energy
- Adding a catalyst
Applications of Chemical Bonds
Chemical bonds are essential for the formation of all matter. They are used in a wide variety of applications, including:
- Construction
- Manufacturing
- Medicine
- Energy
Clarifying Questions
What are the different types of chemical bonds?
The four main types of chemical bonds are covalent bonds, ionic bonds, hydrogen bonds, and metallic bonds.
What are the properties of covalent bonds?
Covalent bonds are formed when two atoms share one or more pairs of electrons. They are typically strong and nonpolar.
What are the applications of chemical bonds?
Chemical bonds are used in a wide variety of applications, including the production of plastics, pharmaceuticals, and fuels.